24/7 Clients Support
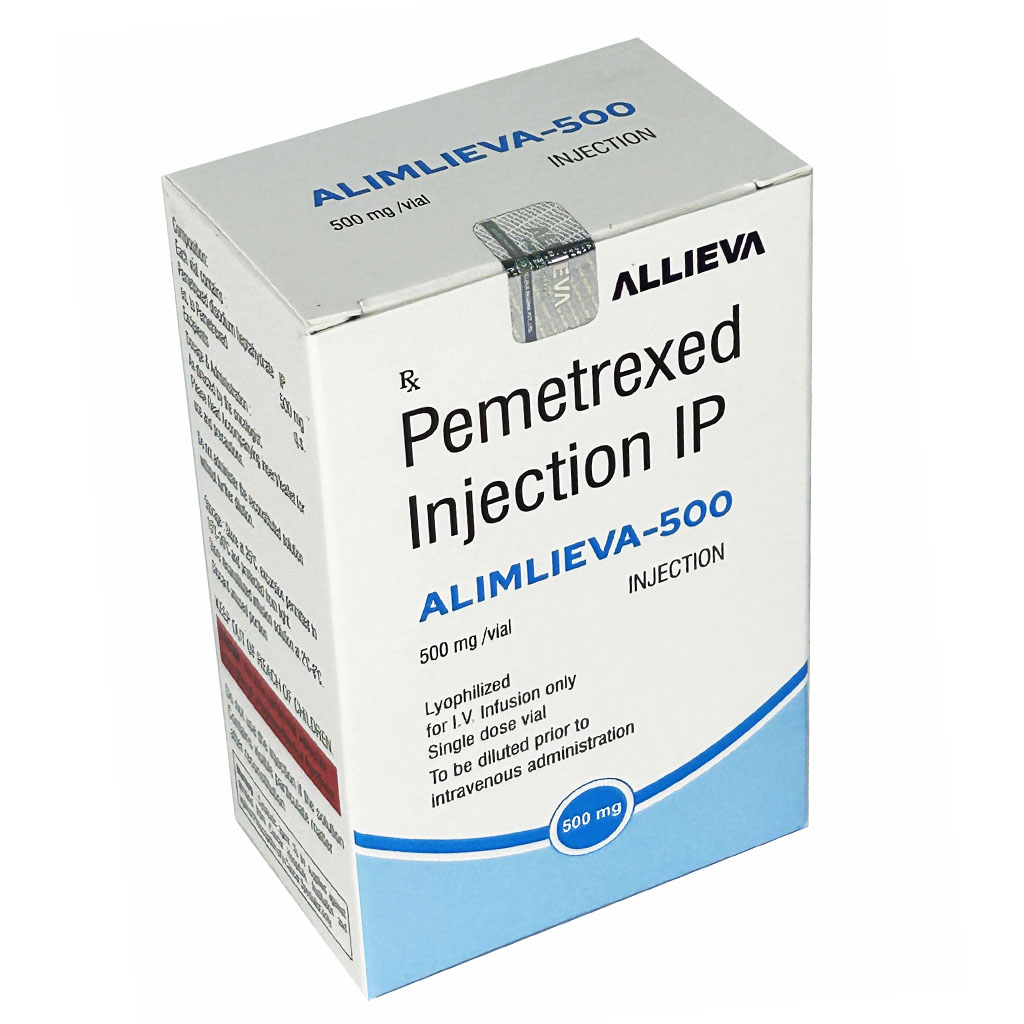

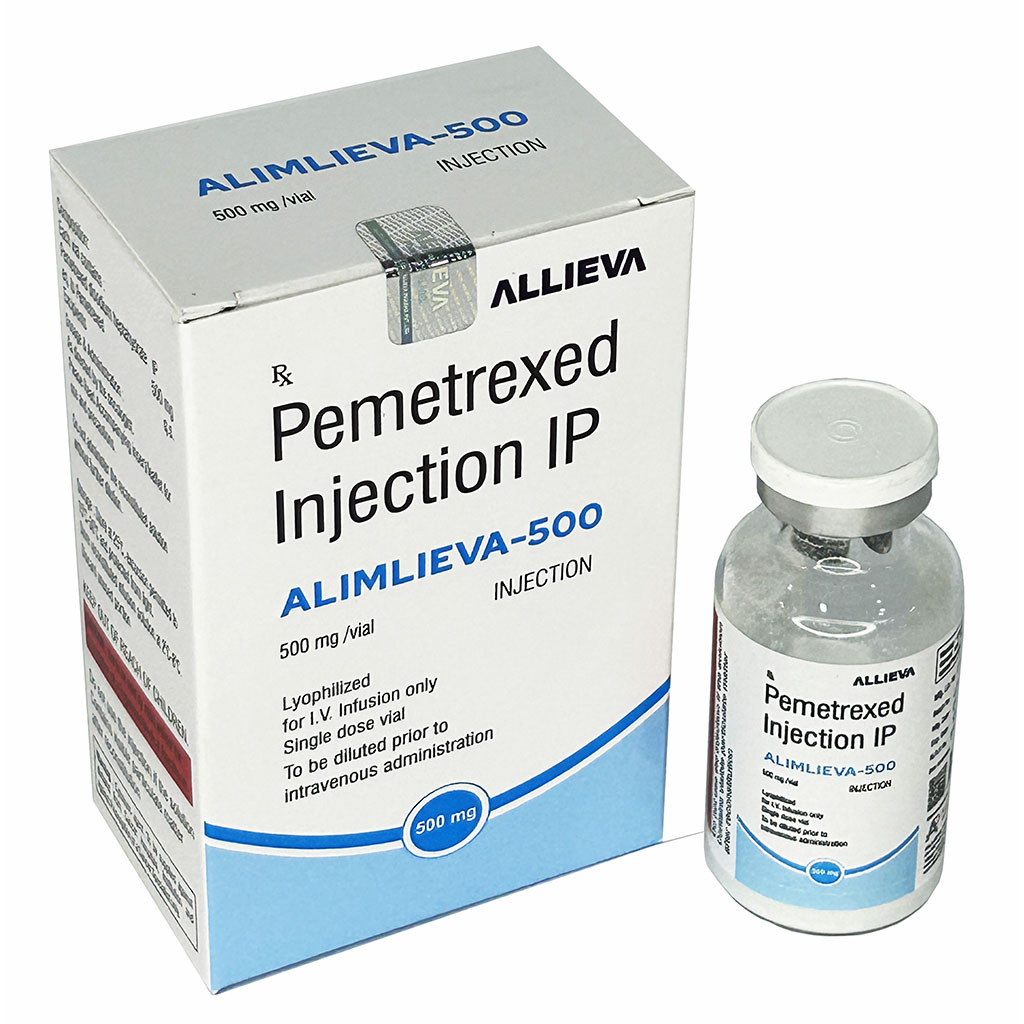
| Brand Name | ALIMLIEVA 500MG |
| Composition | Pemetrexed Injection IP 500mg |
| Manufacture By | Allieva Pharma Private Limited |
| Form | Injection |
| Packing | Vial |
| Country Of Origin | India |
| Description | ALIMLIEVA 500 mg contains Pemetrexed, a chemotherapy (anticancer) drug classified as a multitargeted antifolate antimetabolite. It inhibits key enzymes involved in purine and pyrimidine synthesis, leading to inhibition of DNA and RNA synthesis and cancer cell death. It is mainly used in non-squamous lung cancers. |
| Uses | Pemetrexed Injection is indicated for:
|
| Side Effects | Common side effects: • Fatigue and weakness • Nausea, vomiting • Loss of appetite • Myelosuppression (anemia, neutropenia, thrombocytopenia) • Skin rash • Mouth sores (stomatitis) Serious side effects: • Severe bone marrow suppression • Infections due to low white blood cells • Kidney function impairment • Severe skin reactions Mandatory vitamin supplementation is required to reduce toxicity. |
| Dosage | Dosage is based on body surface area (BSA). Standard dose: • 500 mg/m² IV infusion on Day 1 of a 21-day cycle Administered by intravenous infusion under oncologist supervision. |
